When the World Health Organization declared COVID-19 a pandemic As of March 11, 2020, humans were the one species wherein cases of the disease had been reported. While early genetic analyses indicated Horseshoe bats because the evolutionary host of SARS-CoV-2, the virus that causes COVID-19, no reports have emerged to this point suggesting that the virus could possibly be transmitted from humans to other animal species.
Less than two weeks later, the primary infection of a domestic cat was reported in Belgium – presumably by its owner. In the summer of 2020, there have been news of COVID-19 outbreaks and the following Culling in Mink farms throughout Europe and the fear of comparable demands Culling in North America. People and other animals on and around mink farms have tested positive, raising questions on the potential of a secondary wildlife reservoir for COVID-19. That is, the virus could infect a species apart from the one it got here from, setting off a cycle of transmission.
Researchers have documented this phenomenon of human-to-animal transmission, colloquially known as Spillback or reverse zoonotic transmissionin each domestic and wild animals. Wild animals will be infected either directly by humans or not directly by domestic animals which were infected by humans. This stepping stone effect provides pathogens with recent opportunities to develop and might transform their spread, as will be seen in flu And tuberculosis.
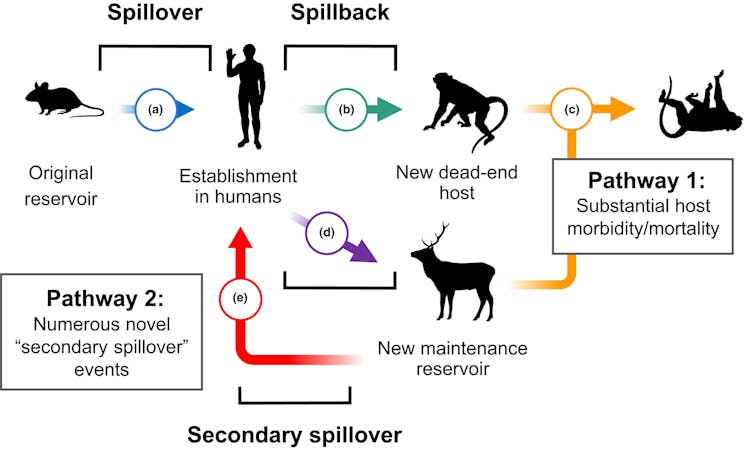
Fagre et al. 2022/Ecology Letters, CC BY-NC-ND
For example, backflow is a long-standing threat to endangered great apeseven amongst populations with rare human contact. The chimpanzees of Gombe National Park, made famous by Jane Goodall's work, have suffered outbreaks of measles and other respiratory diseases, likely brought on by: Persistence of pathogens within the environment Spread by residents or ecotourists.
We are researchers who investigate Mechanisms that drive disease transmission between species And how Disease affects each species conservation and humans. Recent outbreaks have underscored the importance of understanding how threats to wildlife health influence the emergence and spread of zoonotic pathogens. Our research suggests that taking a look at historical outbreaks can assist predict and stop the subsequent pandemic.
Backflow has happened before
Our research group wanted to find out how steadily spillback events were reported within the years before the COVID-19 pandemic. A retrospective evaluation not only allows us to discover specific trends or barriers to reporting spillback events, but in addition helps us understand where recent threats are most certainly to emerge.
We examined historical spillback events involving different groups of pathogens across the animal kingdom, accounting for differences in geography, methods, and sample size. We synthesized scientific reports on spillback for nearly a century before the COVID-19 pandemic – from the Nineteen Twenties to 2019 – which included diseases starting from salmonella and intestinal parasites to tuberculosis, influenza and polio in humans.
In addition, we wanted to find out whether biased evidence and reporting could influence knowledge in regards to the transmission of pathogens from humans to animals. Charismatic megafauna – often defined as larger mammals similar to pandas, gorillas, elephants and whales that evoke emotions in humans – tends to be overrepresented in wildlife epidemiology and species conservation. They receive more public attention and funding than smaller and fewer visible species.
To make matters worse, there are difficulties Monitoring wild small animal populationsbecause they decay quickly and are sometimes eaten by larger animals. This drastically shortens the window of time wherein researchers can investigate outbreaks and collect samples.

Christopher Kimmel/Moment via Getty Images
The results of our historical evaluation confirm our suspicion that the majority reports Outbreaks in large charismatic megafaunaMany have been kept in captivity, similar to in zoos or rehabilitation centres, or in semi-captivity, similar to well-studied great apes.
Despite the big variety of publications on recent pathogens discovered in bats and rodents, there have been few studies on pathogens transmitted from humans to those animals. Small mammals that occupy different ecological niches, including people who live near human habitation – similar to Deer mice, Rats and skunks – they might not only be more prone to pass on their pathogens to other people, but they might also be more easily infected with human pathogens.
COVID-19 and pandemic flu
In our historical evaluation of spillback effects before the COVID-19 pandemic, the one evidence we found for the establishment of a human pathogen in a wild animal population were two reports from 2019 wherein H1N1 infection in striped skunksLike coronaviruses, influenza A viruses similar to H1N1 are adept at switching hosts and might infect a wide selection of species.
In contrast to coronaviruses, nonetheless, their widespread transmission is facilitated by migratory waterfowl like geese and geese. How exactly these skunks became infected with H1N1 and the way long the infection lasted continues to be unclear.
Shortly after we accomplished the evaluation for our study, reports appeared that widespread COVID-19 infection in white-tailed deer across North America occurred in November 2021. In some areas, the prevalence of infection was as much as 80% although there have been hardly any signs of illness within the deer.
This ubiquitous mammal has turn out to be a virtual secondary reservoir of COVID-19 in North America. In addition, genetic evidence suggests that SARS-CoV-2 develops thrice faster in white-tailed deer than in humans, which can increase the danger of latest variants being transmitted to humans and other animals. There is already evidence of Transmission from deer to humans a previously unknown variant of COVID-19.
There are over 30 million white-tailed deer in North Americalots of them in agricultural and suburban areas. Surveillance efforts to watch virus evolution in white-tailed deer can assist discover emerging variants and further transmission from deer populations to humans or domestic animals.
Studies on related species have shown that the danger of reflux varies. White-tailed deer and mule deer are very at risk of COVID-19 within the laboratory, whereas moose usually are not.
H5N1 and the dairy herd within the USA
Since 2022, the spread of H5N1 affects a wide selection of bird species and mammal species all over the world – foxes, skunks, raccoons, opossums, polar bears, coyotes and seals, to call a couple of. Some of those populations are threatened or endangered, and aggressive surveillance efforts are being implemented to watch virus spread.
At the start of the 12 months, the US Department of Agriculture reported the presence of the H5N1 virus within the milk of dairy cows. Genetic analyses indicate that the virus was introduced into cows. already in December 2023probably within the Texas Panhandle. Since then, it has had an impact 178 herds of cattle in 13 states As of August 2024.
How the virus entered the dairy cow population continues to be unclear, but it surely probably happened through Migratory birds infected with the virusEfforts are underway to explain exactly how the virus moves inside and between herds, even though it appears contaminated milking equipment as an alternative of Aerosol transmissioncould possibly be the perpetrator.

Jacob Wackerhausen/iStock via Getty Images Plus
Because influenza A viruses, like avian influenza, can infect a wide selection of species, it’s critical that surveillance efforts focus not only on dairy cows but in addition on animals living on or near affected farms. Monitoring high-risk areas for interspecies transmission, similar to where livestock, wildlife and humans converge, not only provides information on how widespread a disease is in a given population—on this case, dairy cows—but in addition allows researchers to discover susceptible species that come into contact with them.
So far, H5N1 has been detected in several dead animals on affected dairy farms, including Cats, birds and a raccoonFrom August 2024 4 people in close contact with infected dairy cows tested positive, one in all them developed respiratory symptoms. Other wild and domestic animal species remain in danger. Similar Monitoring efforts Studies are currently underway to watch the transmission of H5N1 from poultry to humans.
Humans are only a part of the network
The language often used to explain interspecies transmission fails to capture its complexity and nuances. Given the variety of species which have turn out to be infected with COVID-19 through the pandemic, many scientists are calling for Restriction on the usage of the terms “spillover” and “spillback” because they describe the transmission of pathogens to and from humans. This suggests that diseases and their consequences begin and end in humans.
When researchers view humans as a node in a big network of transmission opportunities, they will more effectively monitor COVID-19, H5N1 and other emerging zoonoses. This involves systems considering Approaches similar to A health or Planetary Health this recording The interdependence of humans on the health of your entire environment.
image credit : theconversation.com

















Leave a Reply