The word magic just isn’t often utilized in a scientific context. But within the early Thirties, scientists discovered that some Atomic nuclei – the central a part of the atoms that make up all matter – were more stable than others. These nuclei had a certain variety of protons or neutrons or magic numbers Physicist Eugene Wigner she called.

Argonne National Laboratory, CC BY-NC-SA
The race to work out what makes these nuclei so stable began. Understanding these magic numbers would allow scientists to predict the properties of other nuclei, similar to their mass or expected lifespan. This would also allow scientists to predict which mixtures of protons and neutrons can form a nucleus.
The solution to the mystery got here from two directions at the identical time in 1949. In the USA, Physicist Maria Goeppert Mayer released an announcement at the identical time a gaggle of scientists led by J. Hans D. Jensen I discovered the identical solution in Germany.

The Nobel Foundation
The two physicists each received 1 / 4 for his or her discovery 1963 Nobel Prize in Physics. We are two Nuclear scientist whose work builds on the discoveries of Goeppert Mayer and Jensen 75 years ago. These magic numbers proceed to play a vital role in our research, only now can we study them in nuclei that only live for a fraction of a second.
Stability within the atom
The atom is a fancy system of particles. It consists of a central nucleus made up of protons and neutrons, called nucleons, orbited by electrons.
Nobel Prize-winning physicist Niels Bohr described that these electrons within the atom are present in a shell structure. The electrons orbit across the nucleus at specific energy levels or orbits. These orbits have specific energies and every orbit can only hold a limited variety of electrons.
Chemical reactions occur through interactions between the electrons in two atoms. In Bohr's modelWhen an electron orbit just isn’t yet filled, it is less complicated for atoms to exchange or share those electrons and trigger chemical reactions.
AG Caesar/Wikimedia Commons, CC BY-SA
A category of elements, the noble gases, hardly react with other elements. In noble gases, the electrons occupy completely filled orbits, and because of this, the atoms greedily hold on to their electrons fairly than share them and undergo a chemical response.
In the Thirties, scientists wondered whether protons and neutrons could occupy orbits similar to electrons. But nobody has been in a position to show this conclusively. For greater than a decade, the scientific community was unable to explain the nucleus when it comes to individual protons and neutrons. Scientists used a simplified picture during which protons and neutrons were treated as a single system, like a drop of water.
Magic numbers
In 1949, Goeppert Mayer and Jensen developed the so-called Shell model of the core. Like electrons, protons and neutrons occupy certain orbits, but in addition they have certain orbits a property called spin – just like a spinning top. Goeppert Mayer and Jensen found that by combining the 2 properties of their calculations they may reproduce the experimental observations.
Through some experiments, they found that nuclei with certain magic numbers of neutrons or protons are unusually stable and hold on to their nucleons more tightly than researchers previously expected, just as noble gases hold on to their electrons.
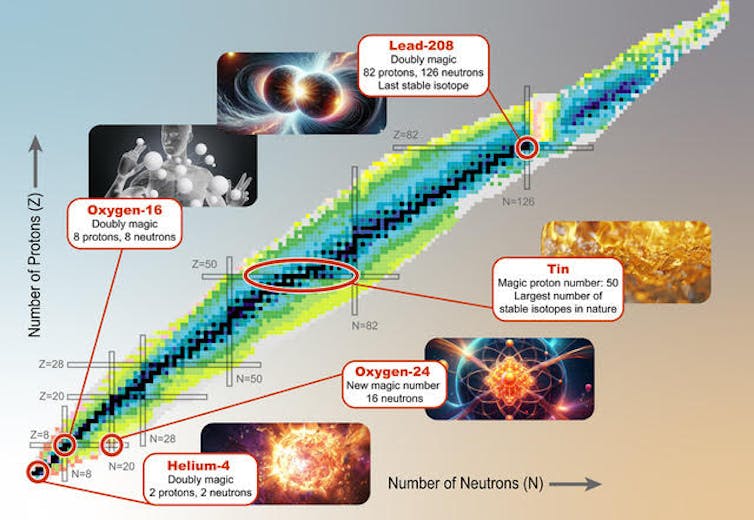
The magic numbers 2, 8, 20, 28, 50, 82 and 126 are known to scientists. They are the identical for protons and neutrons. If a nucleus has a magic variety of protons or neutrons, then the respective orbit is filledand the nucleus just isn’t very reactive, just like the noble gases.
For example, the element tin has a magical variety of protons. Tin at all times has 50 protons and that's it most typical isotope has 70 neutrons. Isotopes are atoms of the identical element which have different numbers of neutrons.
There are nine other stable isotopes of tin that may exist – it’s the element with the biggest variety of stable isotopes. A stable isotope won’t ever spontaneously transform into one other element, which is the case with radioactive isotopes.
With two protons and two neutrons, helium is the lightest “double magic” nucleus. Both its neutron number and its proton number are a magic number. The forces holding the helium-4 nucleus together are so strong that it’s unattainable to connect one other proton or neutron. If you tried so as to add one other proton or neutron, the resulting atom would disintegrate immediately.
On the opposite hand, the heaviest stable core in existence, Lead-208, can also be a double magic core. It has the magic variety of 82 protons and 126 neutrons.
The facility for rare isotope beams
Examples of magic numbers and stable nuclei are in all places – but scientists couldn't explain them without introducing the shell model.
Stable nuclei in nature
The shell structure in nuclei tells researchers how elements are distributed on Earth and within the universe.
One of probably the most abundant elements on our planet and within the human body is oxygenparticularly the isotope oxygen-16.
With eight protons and eight neutrons, oxygen-16 has a particularly stable nucleus. A close-by star produced the oxygen we discover on Earth through nuclear reactions in its core just before the solar system formed.
Because oxygen nuclei are doubly magical, these nuclei didn’t interact very strongly with other nuclei within the star. This left more oxygen, which eventually served as a vital part for all times on Earth.
In her Nobel LectureMaria Goeppert Mayer spoke about her work Physicist Edward Teller. The two had tried to explain how these elements were formed in stars. In the Thirties, they found it unattainable to elucidate why certain elements and isotopes are more common in stars than others. She later found that the increased abundance was linked to nuclei that had something in common: all of them had a magic variety of neutrons.
With the shell model and the reason of magic numbers, the production of elements in stars became possible and was published in 1957.
Scientists today proceed to make use of ideas from the nuclear envelope model to elucidate recent phenomena in nuclear science. Some accelerator facilities, like that Facility for rare isotope beamswhere we work goals to create more exotic nuclei to grasp how their properties change in comparison with their stable counterparts.
At the Facility for Rare Isotope Beams, scientists create recent isotopes by accelerating stable isotopes to about half the speed of sunshine and hurling them at a goal. We select the rarest pieces and examine their properties.
Perhaps probably the most profound modern discovery is the incontrovertible fact that the magic numbers change in exotic cores like the sort created here. So 75 years after the unique discovery, the race to find the subsequent magic number remains to be ongoing.
image credit : theconversation.com


















Leave a Reply