Diabetes occurs when the body fails to manage its blood sugar levels. One type of diabetes causes the body to not produce insulin in any respect. Called Type 1 diabetes or T1DThis autoimmune disease occurs when the body's immune system thinks its own insulin-producing cells are foreign and kills them. On average, T1D could cause this in patients lose a mean of 32 years of healthy life.
Current treatment for T1D includes lifelong insulin injections. Although it’s effective, patients taking insulin are prone to developing low blood sugar levels, which might result in symptoms akin to tremors, irritability, hunger, confusion, and dizziness. In severe cases, seizures or lack of consciousness may occur. Real-time blood glucose monitors and injection devices will help prevent low blood sugar levels by controlling insulin secretion, but they don’t work for some patients.
A treatment is given for these patients Islet transplantation will help higher control blood sugar levels by giving them each recent insulin-producing cells and cells that prevent glucose levels from falling too low. However, it is proscribed by the supply of donors and the necessity to use immunosuppressive drugs. Only about 10% of T1D patients are suitable for islet transplantation.
In my work as Diabetes researchermy colleagues and I even have discovered that producing islet cells from stem cells will help overcome transplant challenges.
History of islet transplantation
Islet transplantation for type 1 diabetes FDA approval in 2023 after greater than a century of research.
Insulin-producing cells, also called beta cells, are positioned within the so-called pancreatic regions Langerhans Islands. They are present in cell clusters that produce other hormones involved in metabolism, akin to: B. Glucagon, which increases blood sugar levels; somatostatin, which inhibits insulin and glucagon; and ghrelin, which signals hunger. anatomist Paul Langerhans discovered islets in 1869, while studying the microscopic anatomy of the pancreas, he found that these cell clusters were markedly different from other cells.
The path to islet transplantation has faced many hurdles since pathologist Gustave-Édouard Laguesse first speculated about it what role islands play in hormone production within the late nineteenth century. In 1893, researchers tried to treat a 13-year-old boy who was dying of diabetes with one Transplanting the pancreas in sheep. While they noted a slight improvement in blood sugar levels, the boy died three days after the procedure.
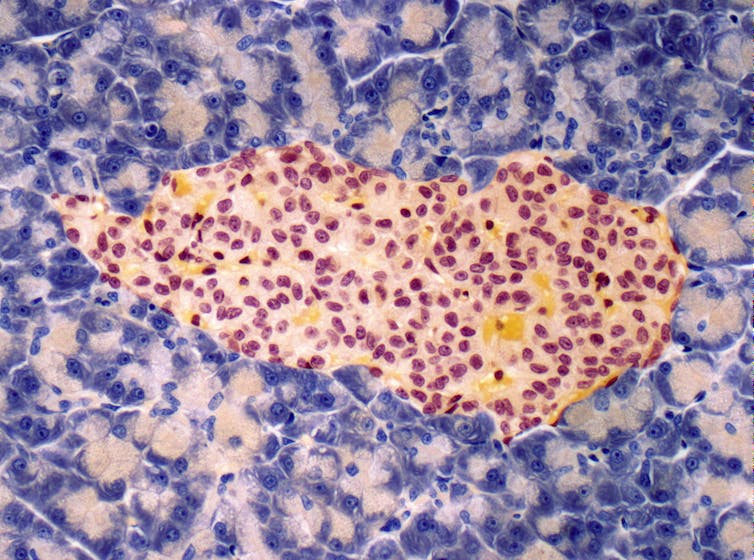
Steve Gschmeissner/Science Photo Library via Getty Images
Interest in islet transplantation was renewed in 1972, when scientists Paul E. Lacy successfully transplanted islets in a diabetic rat. Thereafter, many research groups attempted islet transplantation in humans, with no or limited success.
In 1999, transplant surgeon James Shapiro and his team successfully transplanted islets in seven patients in Edmonton, Canada, by transplanting large numbers of islets from two to 3 donors directly and using immunosuppressive drugs. Through the Edmonton ProtocolThese patients were capable of manage their diabetes without insulin for a 12 months. Until 2012 Over 1,800 patients have undergone islet transplants based on this system, and roughly 90% survived the seven years of follow-up. The first FDA-approved islet transplant therapy based on the Edmonton Protocol.
Stem cells as a source for islets
Islet transplantation is now considered a minor surgical operation by which islets are injected right into a vein within the liver using a catheter. As easy because it could seem, it exists many challenges related to the procedure, including high cost and limited availability of donor islets. The transplant also requires lifelong use of immunosuppressants that allow the foreign islets to live and performance within the body. However, using immunosuppressants also increases the chance of other infections.
To overcome these challenges, researchers are considering using stem cells to create a limiteless source of islets.
There are two forms of stem cells For islet transplants, scientists use: embryonic stem cells, or ESCs, and induced pluripotent stem cells, or iPSCs. Both species can mature into islands within the laboratory.
Each has benefits and downsides.
There are ethical concerns about ES because they’re derived from dead human embryos. ESC transplantation would still require immunosuppressive drugs, limiting their use. Therefore, researchers are working on either encapsulating ESC islands or making mutations to guard them from the body's immune system.
Conversely, iPSCs are obtained from skin, blood or fat cells of the transplant patient. Since the transplant involves the patient's own cells, there isn’t a must take immunosuppressants. But the price of generating iPSC islands for every patient represents a significant obstacle.
Challenges with stem cell islands
While iPSCs could theoretically eliminate the necessity for immunosuppressive drugs, this method has yet to be tested within the clinic.
T1D patients with genetic mutations that cause the disease cannot currently use iPSC islets since the cells that will be used to create stem cells can also carry the identical disease-causing mutation of their islet cells. Many gene editing tools available could possibly remove these mutations and generate functional iPSC islands.
In addition to the challenge of genetic optimization, price is a significant problem in islet transplantation. The transplantation of islet cells from stem cells is dearer than insulin therapy on account of the upper costs Manufacturing costs. Efforts to scale the method and make it less expensive also include creation Biobanks for iPSC matching. This would allow iPSC islets for use for multiple patient, reducing costs by eliminating the necessity to generate freshly modified islets for every patient. Embryonic stem cell islands have the same advantage in that the identical batch of cells may be used for all patients.
There can also be a risk that tumors will form from these stem cell islands after the transplant. Until now, Laboratory studies on rodents And clinical trials on humans rarely suffer from cancer. This suggests that the likelihood of those cells forming a tumor is low.
However, many rounds of research and development are required before stem cell islands may be utilized in the clinic. It's a tricky road, but I consider just a few more tweaks will help researchers beat diabetes and save lives.
image credit : theconversation.com


















Leave a Reply