Many human activities release pollutants into the air, water and soil. These harmful chemicals threaten human health and the ecosystem. According to the World Health Organization, air pollution causes estimated 4.2 million deaths annually.
Scientists are in search of solutions and one possible approach is a Class of materials called photocatalystsWhen these materials are activated by light, they perform chemical reactions that, in accordance with initial studies, Degradation of common toxic pollutants.
I’m a Researcher in materials science and engineering on the University of Tennessee. Using robotics and artificial intelligence, my colleagues and I are developing and testing recent photocatalysts with the goal of reducing air pollution.
Break down pollutants
The photocatalysts work through Generation of charged charge carriers within the presence of sunshine. These charged carriers are tiny particles that may move and trigger chemical reactions. When they arrive into contact with water and oxygen within the environment, they produce substances that reactive oxygen speciesThese highly energetic reactive oxygen species can bind to parts of the pollutants after which either decompose the pollutants or convert them into harmless – and even useful – products.
Astita Dubey
However, some materials utilized in the photocatalytic process have limitations. For example, they will only start the response if the sunshine has enough energy – infrared rays with lower energy light or visible lightwon’t trigger the response.
Another problem is that the charged particles involved within the response can recombine too quickly, meaning they arrive back together before they’ve accomplished their task. In these cases, the pollutants either don’t decompose completely or the method takes an extended time.
In addition, the surface of those photocatalysts can sometimes change during or after the photocatalytic response, affecting their functionality and efficiency.
To overcome these limitations, the scientists in my team try to develop recent photocatalytic materials that efficiently degrade pollutants. We also ensure that that these materials are non-toxic in order that our pollutant cleansing materials don’t cause further pollution.
Astita Dubey
Tiny crystals
The scientists in my team use automated experiments and artificial intelligence to seek out out which photocatalytic materials is perhaps the very best candidates for the rapid degradation of pollutants. We manufacture materials and test them so-called hybrid perovskitesThese are tiny crystals which might be a few tenth as thick as a hair.
These nanocrystals consist of a mix of organic (carbon-based) and inorganic (non-carbon-based) components.
They have some unique properties, resembling their excellent light absorption properties, which arise from their structure on the atomic level. They are tiny, but mighty. They are also visually amazing – they interact with light in fascinating ways, generate large numbers of tiny charge carriers and trigger photocatalytic reactions.
These materials transport electrical charges efficiently, allowing them to move light energy and drive chemical reactions. They are also used to make solar panels more efficient and in LED lights that create the colourful displays you see on television screens.
There are 1000’s of potential sorts of hybrid nanocrystals, so my team desired to work out learn how to quickly create and test as a lot of them as possible to seek out out which of them could be best at eliminating toxic pollutants.
Use of robots
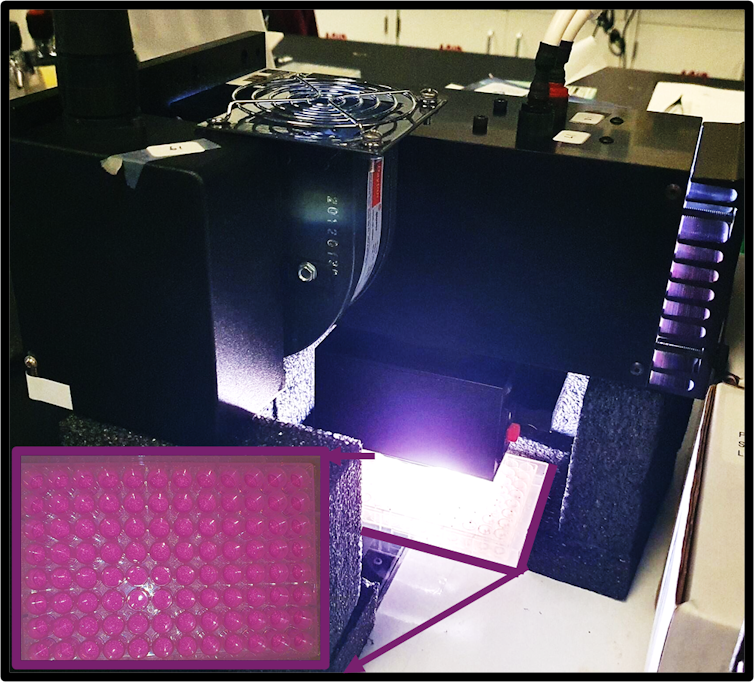
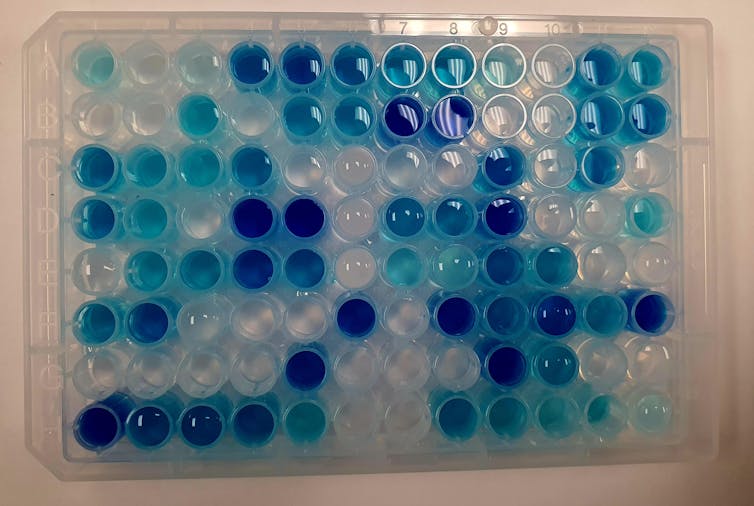
Instead of preparing and testing samples by hand – which might take weeks or months – we use intelligent robotsthat may produce and test no less than 100 different materials inside an hour. These small liquid handling robots can precisely move, mix and transfer tiny amounts of liquid from one place to a different. They are controlled by a pc that controls their acceleration and accuracy.

Jordan Marshall
us too Use machine learning to guide this process. Machine learning algorithms can quickly analyze test data after which learn from that data for the subsequent set of experiments run by the robots. These machine learning algorithms can quickly discover patterns and insights in collected data that may normally take a human eye for much longer to capture.
Our approach goals to simplify and higher understand complex photocatalytic systems to develop recent strategies and materials. By using automated experiments based on machine learning, we will now analyze and interpret these systems more easily, overcoming challenges that were difficult with conventional methods.
image credit : theconversation.com

















Leave a Reply