For many individuals, the smell of freshly brewed coffee is the beginning of a terrific day. But Caffeine could cause headaches and nervousness in others. That's why many individuals reach for a cup of decaffeinated coffee as an alternative.
I’m a chemistry professor who has lectured on why chemicals dissolve in some liquids but not others. The processes of decaffeination provide great real-life examples of those chemical concepts. But even the perfect decaffeination method doesn’t remove all of the caffeine – There are typically about 7 milligrams of caffeine left in a 230 ml cup.
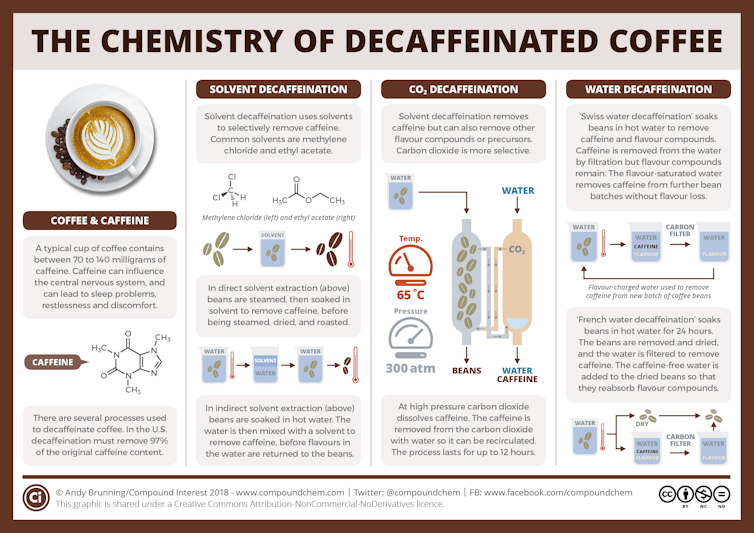
Manufacturers who decaffeinate their coffee wish to remove the caffeine while retaining all – or not less than most – of the opposite chemical aromas and flavors. Decaffeination has a wealthy historyand now just about all coffee producers use certainly one of three common methods.
All these methods, that are also used to make decaffeinated tea, Start with green or unroasted coffee beans which have been pre-moistened. Using roasted coffee beans would lead to a coffee with a totally different aroma and taste, because the Decaffeination steps would remove some flavors and odors is created during roasting.
The carbon dioxide method
In the relatively latest carbon dioxide method, developed within the early Seventies, manufacturers use CO₂ under high pressure to extract caffeine from moistened coffee beans. They pump the CO₂ right into a sealed vessel containing the moistened coffee beans, and the caffeine molecules dissolve within the CO₂.
Once the caffeinated CO₂ is separated from the beans, manufacturers pass the CO₂ mixture either through a container of water or over a bed of activated carbonActivated carbon is carbon that has been heated to high temperatures and exposed to steam and oxygen, creating pores within the carbon. This step filters out the caffeine and more than likely other chemical compoundsa few of which affect the taste of the coffee.
These compounds either bind within the pores of the activated carbon or they continue to be within the water. The manufacturers dry the decaffeinated beans using heat. The heat causes the remaining CO₂ to evaporate. The manufacturers can then pressurize the CO₂ again and reuse it.
This method removes 96% to 98% of caffeineand the resulting coffee has minimal CO₂ residues.
This method, which requires expensive equipment for the production and handling of CO₂, is used extensively to decaffeinate business grade or supermarket coffee.
Swiss Water Process
The Swiss water method, which was initially commercially within the early Nineteen Eightiesuses hot water to decaffeinate coffee.
First, manufacturers soak a batch of green coffee beans in hot water, which extracts each the caffeine and other chemical compounds from the beans.
It's much like brewing roasted coffee beans – you place dark beans in plain water and the chemicals that give the coffee its dark color are leached from the beans into the water. In an analogous way, the new water extracts the caffeine from the undecaffeinated beans.
During the soaking process, the caffeine concentration within the coffee beans is higher than within the water, so the caffeine from the beans goes into the water. Then the manufacturers take the beans out of the water and put them in fresh water that doesn’t contain caffeine – so the method repeats itself, and more caffeine goes from the beans into the water. The manufacturers repeat this process as much as 10 times until there may be hardly any caffeine left within the beans.
The resulting water, which now accommodates the caffeine and any flavors which have dissolved from the beans, is passed through activated carbon filters. These capture caffeine and other chemical compounds similar in size to sugar. and organic compounds, so-called polyamineswhile most other chemical compounds remain within the filtered water.
Manufacturers then use the filtered water—saturated with flavor but nearly caffeine-free—to soak a brand new batch of coffee beans. This step reintroduces the flavors lost through the soaking process into the beans.
The Swiss Water Process is valued for its chemical-free approach and its ability to preserve many of the coffee’s natural flavor. This method Proven to remove 94% to 96% of caffeine.
Solvent-based methods
This traditional and commonest approach, first within the early 1900suses organic solvents, that are liquids that dissolve organic chemical compounds like caffeine. Ethyl acetate and methylene chloride are two common solvents used to extract caffeine from green coffee beans. There are two fundamental solvent-based methods.
In the direct method, manufacturers soak the wet beans directly within the solvent or in a water solution containing the solvent.
The solvent extracts many of the caffeine and other chemical compounds with similar solubility to caffeine from the coffee beans. Manufacturers then remove the beans from the solvent after about 10 hours and dry them.
In the indirect method, manufacturers soak the beans in hot water for a number of hours after which take them out. They then treat the water with a solvent to remove the caffeine. Methylene chloride, essentially the most commonly used solvent, doesn’t dissolve in water, so it forms a layer on top of the water. The caffeine dissolves higher in methylene chloride than in water, so many of the caffeine stays within the methylene chloride layer, which manufacturers can separate from the water.
Andy Brunning/Compound interest, CC BY-NC
As with the Swiss water method, manufacturers can reuse the “caffeine-free” water, which may add back among the flavors removed in step one.
Remove these methods about 96% to 97% of the caffeine.
Is it secure to drink decaffeinated coffee?
One of essentially the most common solvents, ethyl acetate, occurs naturally in lots of foods and beverages. It is taken into account secure chemical for decaffeination from the Food and Drug Administration.
The FDA and the Occupational Safety and Health Administration have classified methylene chloride as unsafe in concentrations above 10 milligrams per kilogram of body weight. However, the quantity of residual methylene chloride in roasted coffee beans is very low – about 2 to three milligrams per kilogram. It is well below FDA limits.
OSHA and its European partners have strict rules within the workplace to attenuate exposure of staff involved within the decaffeination process to methylene chloride.
After the coffee beans are decaffeinated with methylene chloride, they’re steamed and dried. The coffee beans are then roasted at high temperatures. During the steaming and roasting process, the beans grow to be hot enough to evaporate any remaining methylene chloride. Roasting also creates latest flavors from the breakdown of chemicals into other chemical compounds. These give coffee its distinctive taste.
In addition, most individuals brew their coffee at between 87 °C and 90 °Cwhich provides one other opportunity for methylene chloride to evaporate.
Preservation of aroma and taste
It is chemically not possible to remove just the caffeine without also removing other chemical compounds within the beans. Therefore, decaffeination inevitably removes other compounds that contribute to the aroma and flavor of your cup of coffee.
However, some techniques, comparable to the Swiss water process and the indirect solvent method, contain steps that may reintroduce a few of these extracted compounds. These approaches probably cannot return the entire extra compounds to the beans, but they will add among the flavor compounds back.
Thanks to those processes, you’ll be able to enjoy a delicious cup of coffee without caffeine – unless your waiter by accident mixes up the pots.
image credit : theconversation.com

















Leave a Reply