When Victor Ambrose And Gary Ruvkun When they found a brand new molecule within the Nineteen Eighties that they called microRNA, it was an intriguing change from what had been called “microRNA” for a long time central dogma of molecular biology.
Recognized with the 2024 Nobel Prize in Physiology or MedicineAmbros and Ruvkun had identified a brand new form of genetic material that modified researchers' understanding of gene regulation.
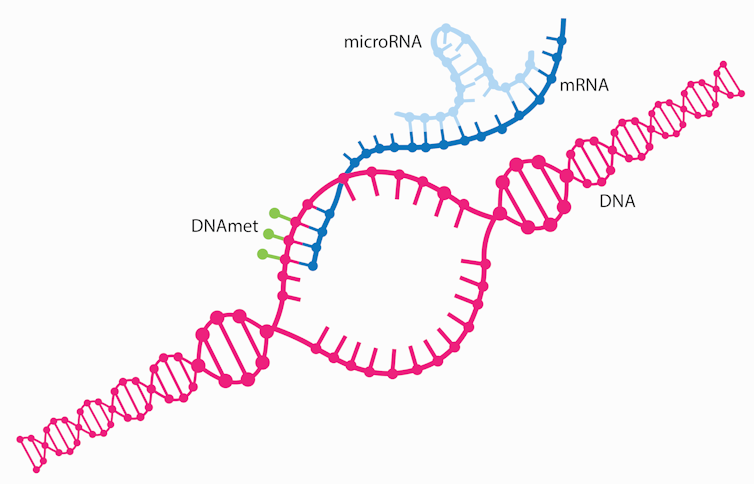
RNA, like DNA, is a type of genetic material that consists of individual nucleotides linked together into chains. According to the central dogma, genetic information flows in a single direction: DNA is transcribed into RNA, and RNA is translated into proteins. But in a significant departure from central dogma, some RNAs are never translated or encoded into proteins.
MicroRNA is a form of these so-called non-coding RNAs. These are short sections of genetic material that don’t themselves code for a particular protein, but fairly control the RNAs that code for proteins. In fact, microRNAs turn certain genes on and off.
I dedicated my scientific profession to know how RNA works, partly because research on RNA lags behind other macromolecules comparable to DNA and proteins. The Nobel Prize recognition for microRNA molecules highlights each their importance in biology and their promise as a possible treatment for various diseases, including cancer.
MicroRNAs and disease
Scientists consider microRNAs as Master regulators of the genome because of their ability to bind to and alter the expression of many protein-coding RNAs. In fact, a single microRNA can regulate between 10 and 100 protein-coding RNAs. Instead of translating DNA into proteins, they’ll as an alternative bind to protein-coding RNAs to silence genes.
The reason microRNAs can regulate such a various pool of RNA is because of their ability to bind to focus on RNAs with which they don’t perfectly match. This signifies that a single microRNA can often regulate a pool of targets, all involved in similar processes within the cell, leading to an enhanced response.
Because a single microRNA can regulate multiple genes, many microRNAs can contribute to disease after they stop functioning.
In 2002, researchers first identified the role of dysfunctional microRNAs within the disease of patients with a particular form of blood and bone marrow cancer chronic lymphocytic leukemia. This cancer results from the Loss of two microRNAs normally involved in blocking tumor cell growth. Since then, scientists have identified over 2,000 microRNAs in humansa lot of that are altered in various diseases.
The field has developed a reasonably solid understanding of how microRNA dysfunction contributes to disease. Changing one microRNA can alter several other genes, leading to a wide range of changes that may overall alter the cell's physiology. For example, greater than half of all cancers show significantly reduced activity microRNA called miR-34a. Because miR-34a regulates many genes involved in stopping cancer cell growth and migration, lack of miR-34a may increase the chance of cancer.
Researchers are considering using microRNAs as a therapeutic against cancer, heart disease, neurodegenerative diseases and others. While the ends in the laboratory were promising, the introduction of microRNA treatments within the clinic was promising overcome several challenges. Many of those are related to inefficient delivery to focus on cells and poor stability, which limits their effectiveness.
Kajsa Mollersen/Wikimedia Commons, CC BY-SA
Delivery of microRNA to cells
One reason it’s difficult to deliver microRNA treatments into cells is that microRNA treatments should be targeted to diseased cells while avoiding healthy cells. Unlike mRNA COVID-19 vaccines, that are taken up by scavenging immune cells whose job is to acknowledge foreign materials, microRNA treatments must trick the body into considering they will not be foreign to avoid an immune attack and change into the intended ones cells to achieve.
Scientists are exploring alternative ways to deliver microRNA treatments to their specific goal cells. One method that’s attracting a whole lot of attention relies on direct use Linking the microRNA with a liganda form of small molecule that binds to certain proteins on the cell surface. Compared to healthy cells, diseased cells can have a disproportionate variety of surface proteins or receptors. Ligands can subsequently help microRNAs reach diseased cells in a targeted manner while avoiding healthy cells. The first ligand approved by the US Food and Drug Administration to deliver small RNAs comparable to microRNAs, N-Acetylgalactosamine or GalNAcdelivers RNAs preferentially to liver cells.
To discover ligands that may deliver small RNAs to other cells, receptors should be found which can be expressed at sufficiently high levels on the surface of the goal cells. Typically, over one million copies per cell are required to attain sufficient delivery of the drug.
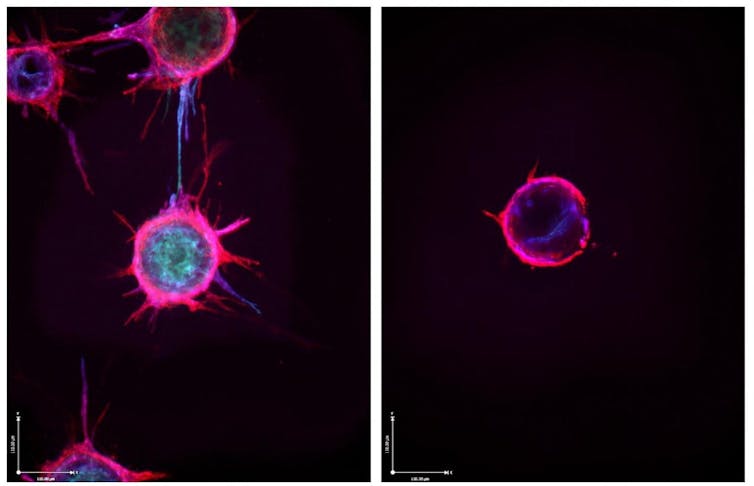
One distinguished ligand is folic acid, also called vitamin B9, a small molecule that’s critical during times of rapid cell growth, comparable to fetal development. Because some tumor cells have one million folate receptors, this ligand provides ample opportunity to deliver enough therapeutic RNA to combat various sorts of cancer. For example, my lab developed a brand new molecule called FolamiR-34a – folate linked to miR-34a – which reduced the dimensions of breast and lung cancer tumors in mice.
Dudley Lab, University of Virginia School of Medicine/NIH via Flickr, CC BY-NC
Making microRNAs more stable
Another challenge with using small RNAs is their poor stabilitywhich ends up in their rapid degradation. Therefore, RNA-based treatments are generally short-lived within the body and require frequent doses to keep up a therapeutic effect.
To overcome this challenge, researchers must Modification of small RNAs in alternative ways. While each RNA requires a particular pattern of modification, successful modifications can significantly increase their stability. This reduces the necessity for frequent dosing, which in turn reduces treatment burden and costs.
For example, modified GalNAc siRNAsone other type of small RNAs, reduces the dosage in non-dividing cells from every few days to once every six months. My team has evolved Folate ligands related to modified microRNAs for cancer treatment, which reduced the dosage from once every other day to once per week. In diseases comparable to cancer, where cells divide rapidly and quickly dilute the delivered microRNA, this increase in activity is a major advance in the sphere. We anticipate that this success will facilitate further development of this folate-bound microRNA as a cancer treatment in the approaching years.
Many laboratories are working to develop treatments based on the discoveries a long time ago by recent Nobel laureates Ambros and Ruvkun. While there continues to be much work to be done to beat the hurdles related to microRNA treatments, it is obvious that RNA holds promise as a therapeutic agent for a lot of diseases.
This is an updated version of an article originally published on November 29, 2023.
image credit : theconversation.com


















Leave a Reply